Practice good housekeeping procedures, such as
the daily removal of trash from spaces.
Practice safety precautions when working with
flammable materials.
Report all potential fire hazards.
Keep firefighting equipment handy and in good
working order.
Ensure closures and fittings are working
properly and report any discrepancies.
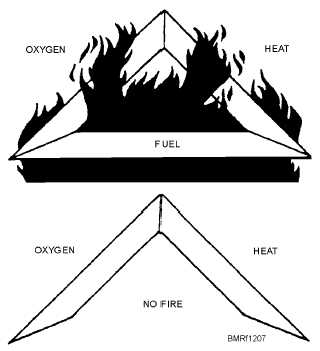
FIRE TRIANGLE
The entire chemistry and physics of fire and
burning, or combustion, can be simplified into a
relationship between three components—fuel, heat
(temperature), and oxygen (air). To have a fire in any
combustible substance, each one of these components
must be present to help each other. Picture these
components in the form of a triangle, as shown in figure
12-7.
Look at figure 12-7. Here, you can see that if the
oxygen reacts with the fuel, it creates heat, which causes
a draft or some other condition that takes in more
oxygen and creates still more heat, and so on. Or the
heat may cause more fuel to become available (such as
causing gasoline to boil into vapor), which then takes
more oxygen to burn and creates more heat, which then
produces still more fuel, and so on. The burning reaction
can go in many different directions.
The modern science of firefighting and fire
extinguishment is based on the sides of the fire triangle
and an uninhibited chain reaction of burning.
Obviously, the firefighter can remove one or more of the
components to cause the burning to stop. The type of
firefighting agent the firefighter has at hand determines
which component or components of the triangle will be
removed.
Another way the firefighter can stop the fire (and the
combustion) is to place a screen between any two
components of the triangle. If the fighter uses an agent
as a temporary screen that breaks the triangle, the fire
goes out. Obviously, the fire can quickly start up again if
this method is used because each of the three necessary
components is still there waiting to start the fire again
once the screen is gone.
FIRE TETRAHEDRON
The fire triangle describes the requirements for
surface glowing or smoldering, but it doesn’t
completely describe flaming combustion requirements.
A fourth requirement, an uninhibited chain reaction, is
needed for flames to exist. This is shown by the fire
tetrahedron (fig. 12-8). A tetrahedron is a solid figure
with four triangular faces. It is useful for illustrating the
flaming combustion process because it provides for the
chemical chain reaction requirement and each face
touches the other three sides. As described for the fire
triangle, flaming combustion stops when one of the four
sides of the fire tetrahedron is removed.
SPONTANEOUS COMBUSTION
Fire, also called burning or combustion, is a rapid
chemical reaction that results in the release of energy in
the form of light and heat. Most spontaneous
combustion involves very rapid oxidation; that is, the
12-19
Student Notes:
Figure 12-7.—Requirements for combustion.